Multi-Mycotoxin Method for Alternaria Toxins, Ergot Alkaloid Epimers, and Other Major Mycotoxins in Food
As more mycotoxins come under regulatory purview, new methods are needed to help food safety labs continue to operate efficiently. Comprehensive, multi-mycotoxin methods are an attractive alternative to separate methods for different analyte lists, but they can be difficult to develop due to the wide range of chemical characteristics among mycotoxin classes. In particular, the Alternaria toxins and ergot alkaloids create additional challenges for method developers. These emerging mycotoxin food contaminants are unique in that, when analyzed on a C18 column, high pH conditions must be used to obtain acceptable peak shape for the Alternaria toxins and adequate separation of the ergot alkaloid epimers. Use of high pH conditions is stressful for LC columns and not suitable for analyzing other classes of mycotoxins, so another approach is required for a truly comprehensive method.

Here, we show the simultaneous analysis of Alternaria toxins, ergot alkaloid epimers, and other major mycotoxins using a simple sample preparation procedure and a Raptor Biphenyl column run under acidic conditions. Under these column- and MS-fri'>ly conditions, accurate quantitative results were obtained in a fast, 11-minute total cycle time run for 37 regulated and emerging mycotoxins (including the Alternaria toxins and all six essential ergot alkaloids and their epimers). In a separate study, method performance was verified in wheat baby cereal, peanut, tomato puree, and BLENDED flour to demonstrate the applicability of this method to a wide range of food types [1'>. Quantitation was performed using matrix-matched standards because isotopically labeled standards were not available for most mycotoxins. Use of this Raptor Biphenyl-based, multi-mycotoxin method provides food safety labs with a means of significantly improving productivity compared to using separate methods for different mycotoxin panels.
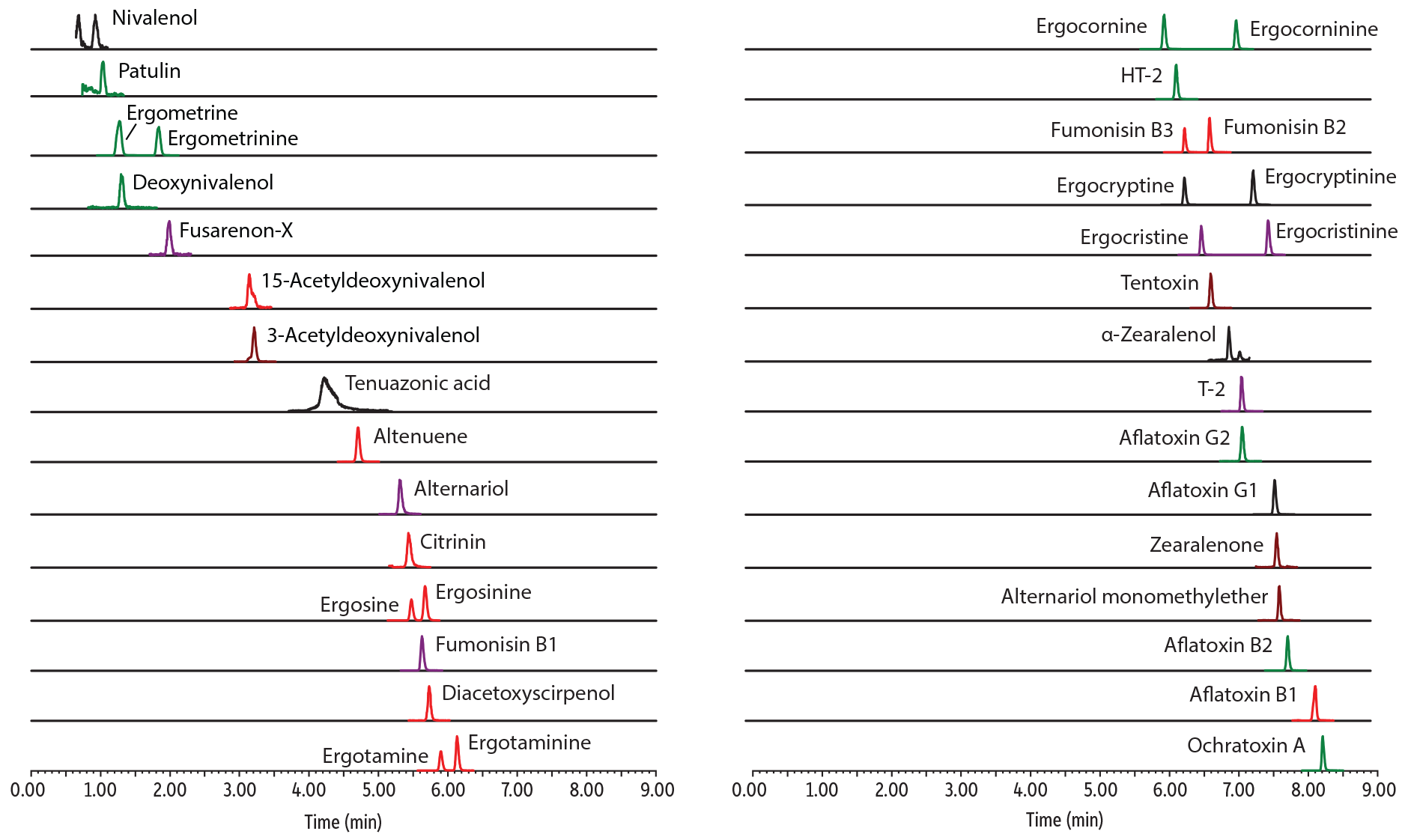
Figure 1: Increase productivity using one multi-mycotoxin method for concurrent analysis of 37 major mycotoxins, including Alternaria toxins and ergot alkaloid epimers (BLENDED flour sample).
| Peaks | tR (min) | Precursor Ion | Product Ion 1 | Product Ion 2 | |
| 1 | Nivalenol | 0.92 | 295.1 | 137.1 | 91 |
| 2 | Patulin | 1.03 | 155 | 99 | 81 |
| 3 | Ergometrine | 1.27 | 326.2 | 223.2 | 208.1 |
| 4 | Deoxynivalenol | 1.3 | 297.2 | 231 | 249 |
| 5 | Ergometrinine | 1.83 | 326.2 | 223.2 | 208.1 |
| 6 | Fusarenon-X | 1.98 | 355.1 | 137.1 | 247.1 |
| 7 | 15-Acetyldeoxynivalenol | 3.14 | 339.2 | 137.1 | 321.2 |
| 8 | 3-Acetyldeoxynivalenol | 3.21 | 339.2 | 213.1 | 231.1 |
| 9 | Tenuazonic acid | 4.22 | 198.1 | 125 | 153.1 |
| 10 | Altenuene | 4.7 | 293.2 | 257.1 | 275.2 |
| 11 | Alternariol | 5.3 | 259 | 185.1 | 130 |
| 12 | Citrinin | 5.43 | 251.2 | 233.1 | 205.1 |
| 13 | Ergosine | 5.47 | 548.4 | 208.1 | 223.2 |
| 14 | Fumonisin B1 | 5.63 | 722.5 | 352.3 | 334.2 |
| 15 | Ergosinine | 5.67 | 548.4 | 208.1 | 223.2 |
| 16 | Diacetoxyscirpenol | 5.73 | 384.2 | 247.1 | 307.2 |
| 17 | Ergotamine | 5.9 | 582.4 | 223.2 | 268.2 |
| 18 | Ergocornine | 6.03 | 562.4 | 268.2 | 223.2 |
| 19 | Ergotaminine | 6.13 | 582.4 | 223.2 | 268.2 |
| 20 | HT-2 | 6.2 | 447.2 | 345.1 | 285.1 |
| 21 | Fumonisin B3 | 6.32 | 706.4 | 336.2 | 318.3 |
| 22 | Ergocryptine | 6.32 | 576.4 | 268.2 | 223.2 |
| 23 | Ergocristine | 6.56 | 610.4 | 223.2 | 592.4 |
| 24 | Fumonisin B2 | 6.68 | 706.4 | 336.2 | 318.3 |
| 25 | Tentoxin | 6.7 | 415.2 | 312.2 | 302.2 |
| 26 | α-Zearalenol | 6.96 | 303.1 | 285.1 | 175 |
| 27 | Ergocorninine | 7.07 | 562.4 | 268.2 | 223.2 |
| 28 | T-2 | 7.14 | 489.2 | 387.1 | 245.1 |
| 29 | Aflatoxin G2 | 7.15 | 331.2 | 189 | 313 |
| 30 | Ergocryptinine | 7.31 | 576.4 | 268.2 | 223.2 |
| 31 | Ergocristinine | 7.53 | 610.4 | 223.2 | 592.4 |
| 32 | Aflatoxin G1 | 7.62 | 329.1 | 199.7 | 243 |
| 33 | Zearalenone | 7.65 | 319.2 | 283.1 | 187 |
| 34 | Alternariol monomethylether | 7.69 | 273 | 199.1 | 128 |
| 35 | Aflatoxin B2 | 7.81 | 315.1 | 287 | 259 |
| 36 | Aflatoxin B1 | 8.2 | 313.2 | 241.1 | 284.9 |
| 37 | Ochratoxin A | 8.31 | 404.1 | 239 | 358 |
| Column | Raptor Biphenyl (cat.# 9309A12) | ||||||||||||||||||||||||
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||
| Guard Column: | Raptor Biphenyl EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9309A0252) | ||||||||||||||||||||||||
| Temp.: | 60 °C | ||||||||||||||||||||||||
| Standard/Sample | Aflatoxins standard (cat.# 34121) | ||||||||||||||||||||||||
| Ochratoxin A standard (cat.# 34122) | |||||||||||||||||||||||||
| Diluent: | 50:50 Water:methanol | ||||||||||||||||||||||||
| Conc.: | 6.25 ng/mL final concentration after sample preparation | ||||||||||||||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||
| A: | Water, 0.05% formic acid | ||||||||||||||||||||||||
| B: | Methanol, 0.05% formic acid | ||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
Detector
|
MS/MS | ||||||||||||||||||||||||
| Ion Mode: | ESI+ | ||||||||||||||||||||||||
| Mode: | MRM | ||||||||||||||||||||||||
| Instrument | UHPLC | ||||||||||||||||||||||||
| Sample Preparation | A BLENDED flour was prepared by mixing white rice flour (75%); brown rice flour (5%); millet flour (5%); oat flour (5%); all-purpose wheat flour (5%); and all-purpose, gluten-free flour (5%). Two grams of the flour sample were weighed into a 50-mL polypropylene centrifuge tube (cat.# 25846) and fortified at 50 µg/kg for all analytes with a stock standard solution. After sitting at room temperature for 10 minutes, 16 mL of extraction solution (80:20 acetonitrile:water) containing 0.5% formic acid were added, and the tube was stirred to create a homogenous suspension. The extraction was carried out by shaking horizontally on a digital pulse mixer (Glas-Col LLC, Terre Haute, IN) at 800 rpm for 20 minutes. After centrifuging for 5 minutes at 4000 rpm, 1 mL of extract was evaporated to dryness at 45 °C under a gentle stream of nitrogen. The dried extract was reconstituted with 1 mL of 50:50 water:methanol solution, and a 0.4 mL aliquot was transferred to and filtered using a Thomson SINGLE StEP filter vial with a 0.2 µm PTFE filter (cat.# 25874). Five µL of the filtered solution was injected for the LC-MS/MS analysis. | ||||||||||||||||||||||||
| Notes | The chromatogram shows peaks with the MS transition of product ion 1. Note that method development work demonstrated that whenever a new column is installed it must be rinsed and maintained under mobile phase overnight to ensure an acceptable peak shape for tenuazonic acid. |
References
H. Liang, J. York, J. Konschnik, H. Majer, J. Steimling, Comprehensive mycotoxin analysis: simultaneous determination of Alternaria toxins, ergot alkaloid epimers, and other major mycotoxins in various food matrices by LC-MS/MS, Restek Corporation, 2022.